Cover art stories
Small (2024) 20, 2403028
The authors present a milled composite based on CuO and CeO2 prepared via-mechanochemical approach. The mechanical energy provided by the synthesis demonstrated the ability to generate Ce4+/Cu+ interfaces which resulted active in the activation of methane into partially oxidized intermediates at the temperature of 250 °C.
J. Am. Chem. Soc. (2024) 146, 25986-25999
Milled Pd-CeO2, with a unique interface composed of Pd-iC-CeO2, enables the conversion of CH4 into CH3OH at a rate of 117 μmol/gcat in peroxide and water at 75 °C. Experiment and theory reveal that the role of interfacial carbon is to enable unique chemistry for the 100% selective transformation of methane into methanol.
ACT EST Water 4 (2024) 2057
The main aim of this study is the development of a fast and simple method for a preliminary evaluation of residual toxicity after treatment of liquid waste, reserving toxicity tests on organisms only for the most promising treatments. The proposed tool is intended to be used for screening analyses. Waterdrop image by macrovector on Freepik.
EES Catalysis 1 (2023) 144
Pd entities supported on ceria by a dry milling method maintain a peculiar configuration with a balanced ratio between reduced Pd0 and oxidized Pd2+ species, leading to high turnover in CH4 oxidation and improved resistance to steam deactivation even under low oxygen availability.
ACS Catalysis 12 (2022) 12809
Pd entities on ceria generated by mechanochemical milling are able to tune DRM selectivity by easier hydrogen and/or carbon doping into the metal lattice.
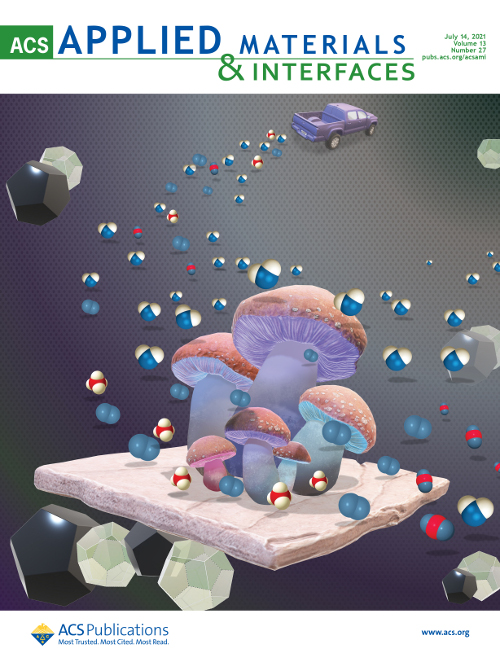
ACS Appl. Mater. Interfaces, 13 (2021) 31614
The large amount of H2O present in exhaust gases of natural-gas-fueled vehicles hinders unburned CH4 abatement by Pd-based catalysts. Here, mushroom-like structures formed at the Pd–ceria interface of bimetallic catalysts obtained by solventless milling of Pd, Pt, and CeO2 powders are observed for the first time, evolving under a "wet" reaction atmosphere and ensuring high activity and stability with an opportune Pd:Pt ratio and milling order.
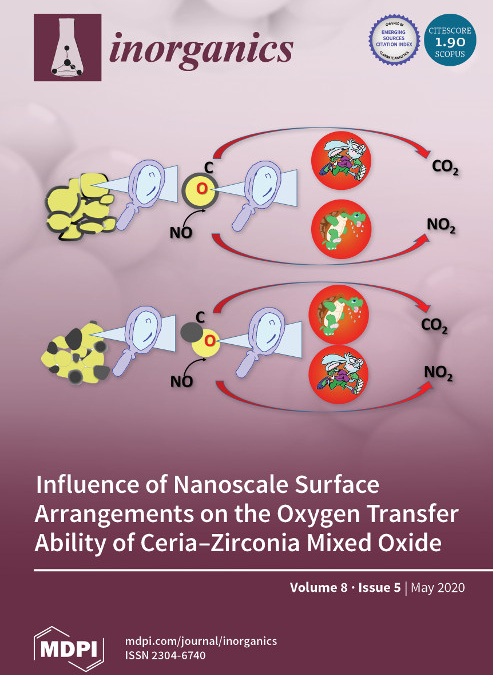
Inorganics, 8(5) (2020) 34
CeO2-ZrO2 solid solutions are key ingredients in catalyst formulations due to the ability of ceria to easily cycle its oxidation state. Here, carbon soot was used as a solid reductant to better understand the oxygen transfer ability of ceria-zirconia at low temperatures in the presence of oxygen and NO/NO2. The existence of a ceria-zirconia core and carbon shell architecture strongly enhanced reactivity of interfacial oxygen species while negatively affecting NO oxidation characteristics. The study confirmed the key role of active oxygen species in soot oxidation with ceria-based materials, and more importantly it disclosed the subtle but distinct redox chemistry of ceria with O2 and NO/NO2 mixtures at varying degrees of interaction with carbon.
Catalysis Science and Technology, 9 (2019) 4232
The preparation of catalytic materials via mechanochemical routes is a very promising alternative to complex wet chemical syntheses due to its simplicity, versatility and ecological advantages. The mechanical mixing of Pd nanoparticles and CeO2 results in very active methane oxidation catalysts; here we explore the effect of milling parameters on the overall performance of Pd–ceria catalysts. The high methane combustion activity is the result of nanoscale interaction between palladium and cerium oxide and it is shown to be strongly dependent on the intensity of milling. This was investigated through methane combustion tests up to 1173 K and characterized by means of temperature programmed oxidation and reduction experiments. The morphological features of the obtained materials were investigated by HRTEM analysis and correlated to the catalytic behavior.
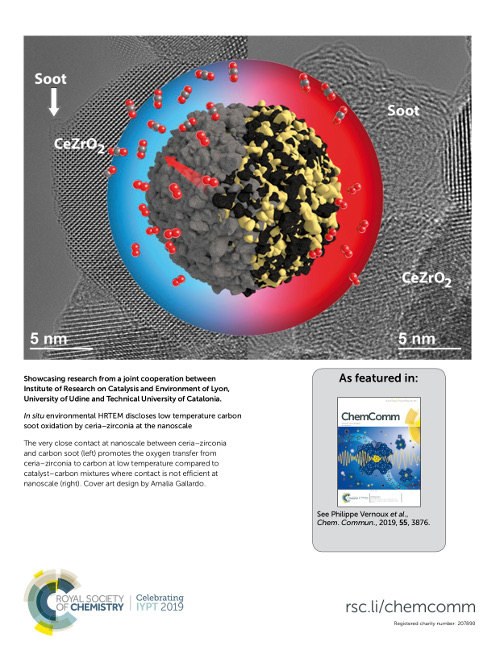
Chemical Communications, 55 (2019) 3876
The very close contact between ceria–zirconia and carbon soot allows the detection of oxygen transfer from ceria–zirconia to carbon by in situ Environmental HRTEM already at low temperatures. This highlights the outstanding redox behavior and soot oxidation potential of ceria–zirconia when suitable carbon–catalyst arrangements are generated at the nanoscale.
Inorganics, 6(2) (2018) 39
In the context of the development of a circular economy based on the use of renewable resources and efficient energy conversion services, ceria zirconia catalysts have been investigated for the valorization of the greenhouse gases, CH4 and CO2 to obtain syngas (CO/H2) for industrial applications and for use as fuel in solid oxide fuel cells (SOFC). The solid solution Ce0.80Zr0.2O2 when doped with Nd is a suitable support for obtaining Ni-based dry reforming catalysts highly resistant to carbon deposition. Ce and Nd play an important role in the function of carbon removal while Zr promotes methane activation.
Angew. Chem. Int. Ed. 56 (2017) 375
Flat ceria (110) surfaces undergo restructuring at high temperatures and expose {111} terraces. This faceting also occurs on ceria nanorods, as demonstrated by Y. Wang, C. Wöll, and co-workers in their Communication on page 379 ff. through IR spectroscopic analysis of adsorbed probe molecules. Alternating flat and corrugated surfaces, here symbolized by a view of the Monument Valley Navajo Tribal Park, provide a likely explanation for the high catalytic activity of rod-shaped ceria nanoparticles.
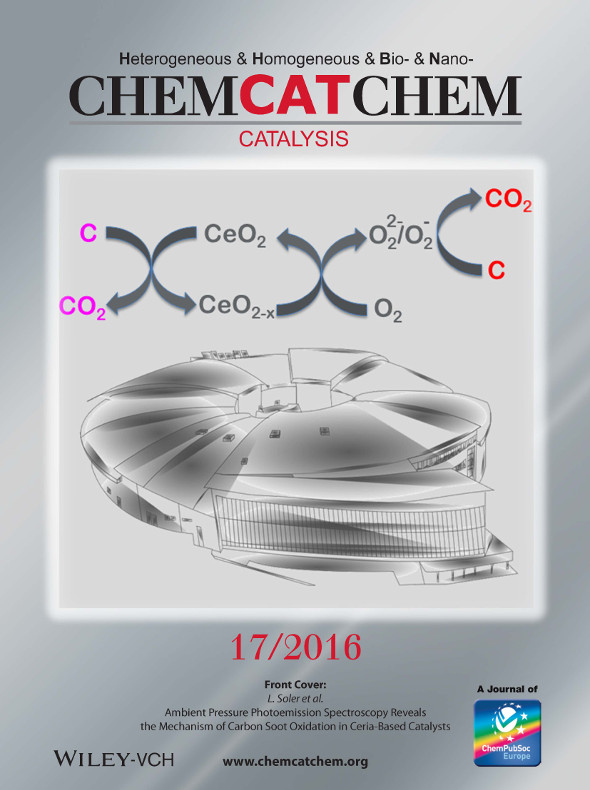
ChemCatChem, 8 (2016) 2748
The Front Cover shows a representation of the ALBA synchrotron, where near ambient pressure photoelectron spectroscopy was performed to study the surface/subsurface of ceria and ceria-zirconia catalysts for removing soot from combustion engines. In their Communication, L. Soler et al. identified two cooperative routes, one occurring at the ceria–soot interface with formation of O vacancies and CeIII, and another at the surface of soot mediated by superoxide species resulting from the reaction between O2 gas and O vacancies (drawing by Javier Sánchez Ríos, javier. sanchezrios.1978@ieee.org). More information can be found in the Communication by L. Soler et al. on page 2748 in Issue 17, 2016.
Catalysis Science and Technology, 6 (2016) 399
Ce0.15Zr0.85O2 was investigated in the two-step water splitting reaction. High-temperature treatment in N2 induced compositional and structural heterogeneities which contributed to a six-fold increase of H2 yield after the first cycle. Ceria surface enrichment and the formation of a ceria–zirconia oxynitride phase positively affected the reduction and oxidation steps.
Angew. Chem. Int. Ed. 53 (2014) 12069
Structure–performance relationshipsof ceria in heterogeneous reactions are revealed by J. Pérez-Ramírez, A. Trovarelli, et al. in their Communication on page 12069 ff. The (111) surface prevalent in conventional polyhedral CeO2 particles outperforms in hydrogenation, while the (100) surface that is exposed in nanocubes dominates in oxidation. Artwork: Amalia Gallardo (ArteLi) and Marcel Reich.